WashU has a pre-award office to advise and assist researchers with proposal preparation. The Office of Sponsored Research Services (OSRS) provides communication, education, and assistance related to proposal development for the WashU research community.
The information below contains information relevant to preparing and submitting proposals including institutional data, grant writing resources, and submission information. For information specific to Principal Investigators, please visit the Proposal Preparation for Faculty page.
For sponsored projects transferring from another institution to WashU, see the Transferring Sponsored Research to WashU Admin Guide.
Proposal Timeline
The process to submit a proposal can take six months or longer, and requires the involvement of the PI, department administrators, and central offices.
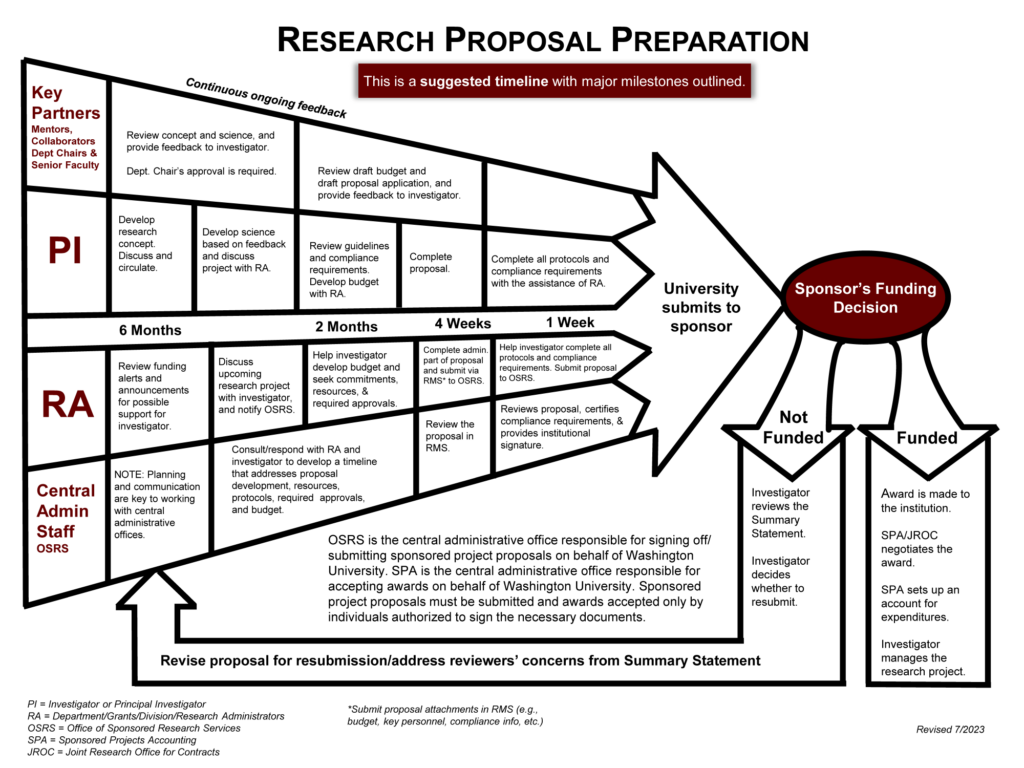
Getting Started: Identify Key Information
The first step in identifying what information is needed for the proposal is to download the Funding Opportunity Announcement (FOA) from the sponsor.
Confirm PI Eligibility
- School of Medicine PI Eligibility Matrix
- WashU Schools outside the School of Medicine have their own policies regarding PI eligibility. Contact your School for their current policies.
- If the proposal is for a limited competition, verify the PI has completed the necessary approvals through Internal Selections.
Due Date
After identifying your proposal’s deadline, plan to submit the proposal in consideration of the RMS Deadline Date Timeline.
Subaward
Submission Method
Login early to verify all forms and requirements.
- FedConnect: lists opportunities for federal contracts, grants, and other types of funding.
- Grants.gov: search for grants and apply via Workspace for grants from over 20 federal agencies including NIH, NSF, DOE, DOD, and DHS.
- eRA Commons: A system managed by NIH that allows applicants, recipients and federal staff to securely share, manage and process grant-related information.
- Application Submission System & Interface for Submission Tracking (ASSIST): is used to prepare and submit applications electronically to NIH and other Public Health Service agencies.
F&A
Use the Facilities & Administrative Rate Flow Chart or Program Types & Purpose Codes to assist in determining the F&A rate for your project.
Understanding Costs of Federally Sponsored Research infographic from COGR.
Budget Considerations
The Uniform Guidance imposed a variety federal requirements detailed in these Budget Considerations.
NSF Proposal Application Checklist
The NSF Proposal Application Checklist outlines the unique elements required for NSF proposals, emphasizing sponsor-specific documents, attachments, and logins required for Senior/Key Personnel.
Should you contact the Joint Research Office for Contracts (JROC)?
- When a RFP anticipates a FAR Contract, you should include JROC in the proposal process.
- JROC should be contacted when an RFP has unusual terms and conditions such as:
- Restrictions on publications
- Export controls/Restrictions on foreign nationals
- Indemnification
- Restrictions on university’s IP rights
- Excessive reporting or invoicing
- Excessive information security requirements
- Accepting Terms & Conditions at proposal stage
- FAR/DFAR/HHSAR Clauses
- Helpful links for JROC and research contracts:
Research Management System
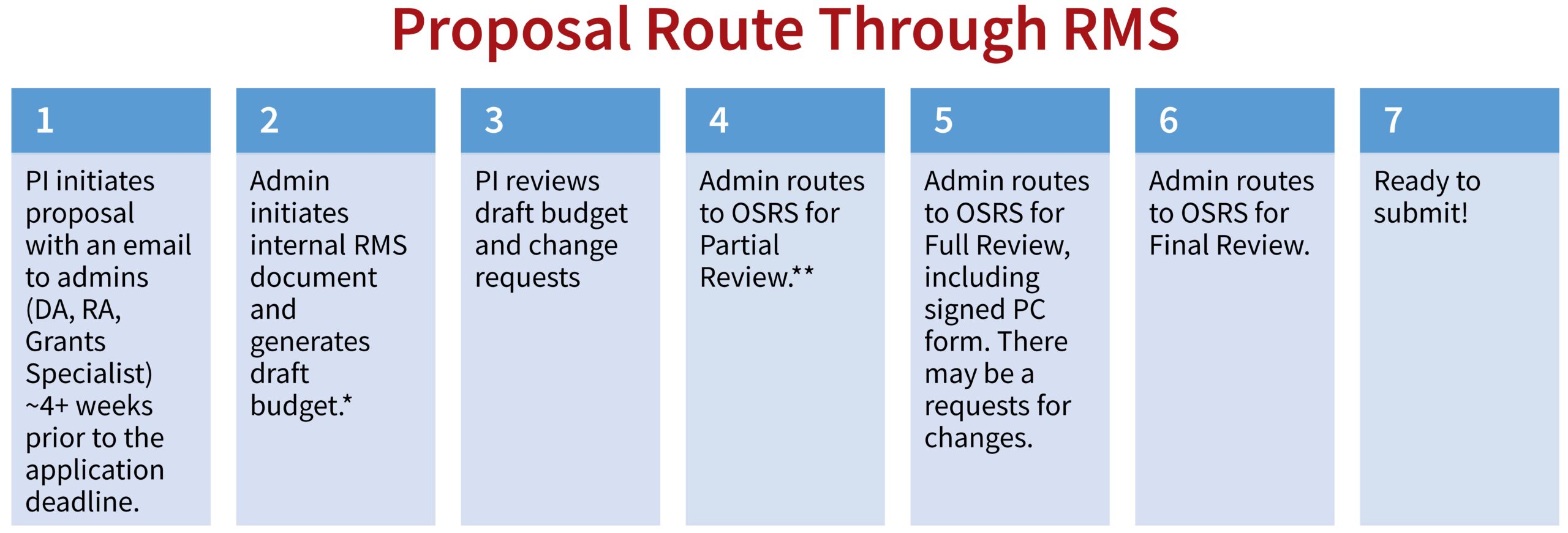
*All proposals (excluding pre-proposals) must go through RMS and the approval process. Begin this process early, see timeline below.
**Once the budget meets the PI’s approval, admin routes to the central Office of Sponsored Research Services (OSRS) for Partial Review. OSRS may approve or request changes. Admin makes requested changes in consultation with PI (if necessary).
OSRS recommended timeline for review:
- 10+ business days for Partial Review
- 5+ business days for Full Review
- 2+ business days for Final Review
RMS User Guide
This guide provides extensive details and step-by-step instructions on how to use RMS.
RMS Resources for Proposal Development
Submitting Information in RMS
This comprehensive list of What to Submit to OSRS includes details of what should be submitted via RMS for S2S NIH grants, S2S non-NIH grants (e.g. DoD, DoE), and non-S2S grants (e.g. CDI, BJHF).
The minimum requirements for what you should submit in RMS to begin the routing process includes:
- Sponsor guidelines or solicitation
- Final budget and budget justifications
- Signed PC form (by all PIs and contact PI’s Chair)
Required information to finalize RMS routing and approvals:
- Complete application
For proposals with subawards:
These two items are required from proposed subrecipients (outgoing subawards). If WashU is the subrecipient (incoming subawards), OSRS will sign the letter of intent.
- Statement/scope of work
- Letter of intent to establish consortium, signed by the sub-institution
Other Documents
Letters of support or collaboration
A letter of support can come from a partner organization, major donor, another foundation, congressional representative, business or other key stakeholders. It provides a compelling and persuasive reason why a funder should support your grant application or proposal.
While letters of collaboration are permitted, unless required by a specific program solicitation, letters of support should not be submitted as they are not a standard component of an NSF proposal. NSF provides a recommended format for letters of collaboration.
Biosketch
NIH Biosketch
- Personal statement – should be specific to the project
- Positions, scientific appointments, and honors
- Contributions to science – do not exceed limit of contributions or number of publications listed
- Link to publications in MyBibliography
NSF Biosketch
The NSF-approved formats include the NSF Fillable PDF or SciENcv
- SciENcv is required for new proposals submitted on or after October 23, 2023
- Page limit: 3 pages
- All senior personnel must provide their bio for the proposal
- Professional Preparation – reverse chronological order by start date
- Appointments – All appointments (adjunct, visiting, and honorary included) in reverse chronological order
- Products – may all be publications: 10 max
- Synergistic Activities – 5 distinct bullets, not paragraphs
Pre-Award Compliance
See Trainings for Researchers for a comprehensive list of major compliance trainings for researchers at WashU.
- Financial Disclosures & External Professional Activities
Prior approval is likely required for higher risk activities that may pose a conflict of commitment. - International Collaborators
Researchers at WashU are obligated to report on international research and scholarly activities. Most international collaborations are not problematic and are encouraged, but researchers must disclose and be transparent regarding their involvement in activities of this nature. - Controlled Unclassified Information (CUI)
If a proposal anticipates the use of CUI, then adequate cybersecurity measures must be in place to accept the contract.
Just-in-Time (JIT)
eRA Commons allows for submitting grant application information requested by the sponsor. A JIT request occurs after the completion of peer review when a project receives a ranking where funding may be possible.
The JIT Procedure in eRACommons involves submitting information and documents in a short period of time. This is done by the PI (or their designee), Department Administrators, and members of OSRS. The information requested my include: